75+ pages consider the following potential sources of error in the titration 2.3mb solution in Google Sheet format. Rinse the buret labeled HCl with about 5 mL of this solution. The first section is a detailed look at how to determine the most important errors. Obtain about 60 mL of 0100 M HCl solution. Check also: consider and consider the following potential sources of error in the titration N if it would have no effect on your value.
Titration is a method used to measure the concentration of a particular solute in a solution. It is type of volumetric quantitative analysis the use of volume measurements to analyze an unknown.

Chemistry Titrations University Of Birmingham
| Title: Chemistry Titrations University Of Birmingham Consider The Following Potential Sources Of Error In The Titration |
| Format: Doc |
| Number of Views: 3440+ times |
| Number of Pages: 215+ pages |
| Publication Date: August 2019 |
| Document Size: 1.7mb |
| Read Chemistry Titrations University Of Birmingham |
 |
A There was a little distilled water in the Erlenmeyer flask before you began the titration.
19 There is a particular risk of errors. Consider each of the following potential error sources. H if it would have caused your calculated value for M HCl to come out too high L if it would have caused it to come out too low or N if it would have had no effect at all on your value. Classic Titration Lab. Using the same pipette for different solutions. 25 26 Errors are more likely to occur when tasks are carried out after hours by busy distracted staff often in relation to unfamiliar patients.
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: Google Sheet |
| Number of Views: 3290+ times |
| Number of Pages: 344+ pages |
| Publication Date: June 2018 |
| Document Size: 1.35mb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: PDF |
| Number of Views: 3270+ times |
| Number of Pages: 211+ pages |
| Publication Date: October 2019 |
| Document Size: 1.2mb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |

Mini Titrator For Measuring Titratable Alkalinity In Water And Wastewater Hi84531u 01
| Title: Mini Titrator For Measuring Titratable Alkalinity In Water And Wastewater Hi84531u 01 Consider The Following Potential Sources Of Error In The Titration |
| Format: PDF |
| Number of Views: 9137+ times |
| Number of Pages: 244+ pages |
| Publication Date: December 2019 |
| Document Size: 1.8mb |
| Read Mini Titrator For Measuring Titratable Alkalinity In Water And Wastewater Hi84531u 01 |
 |

Titration Techniques In The Food Industry New Food Magazine
| Title: Titration Techniques In The Food Industry New Food Magazine Consider The Following Potential Sources Of Error In The Titration |
| Format: Google Sheet |
| Number of Views: 9160+ times |
| Number of Pages: 159+ pages |
| Publication Date: September 2019 |
| Document Size: 1.4mb |
| Read Titration Techniques In The Food Industry New Food Magazine |
 |
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: PDF |
| Number of Views: 8161+ times |
| Number of Pages: 91+ pages |
| Publication Date: July 2019 |
| Document Size: 3mb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |
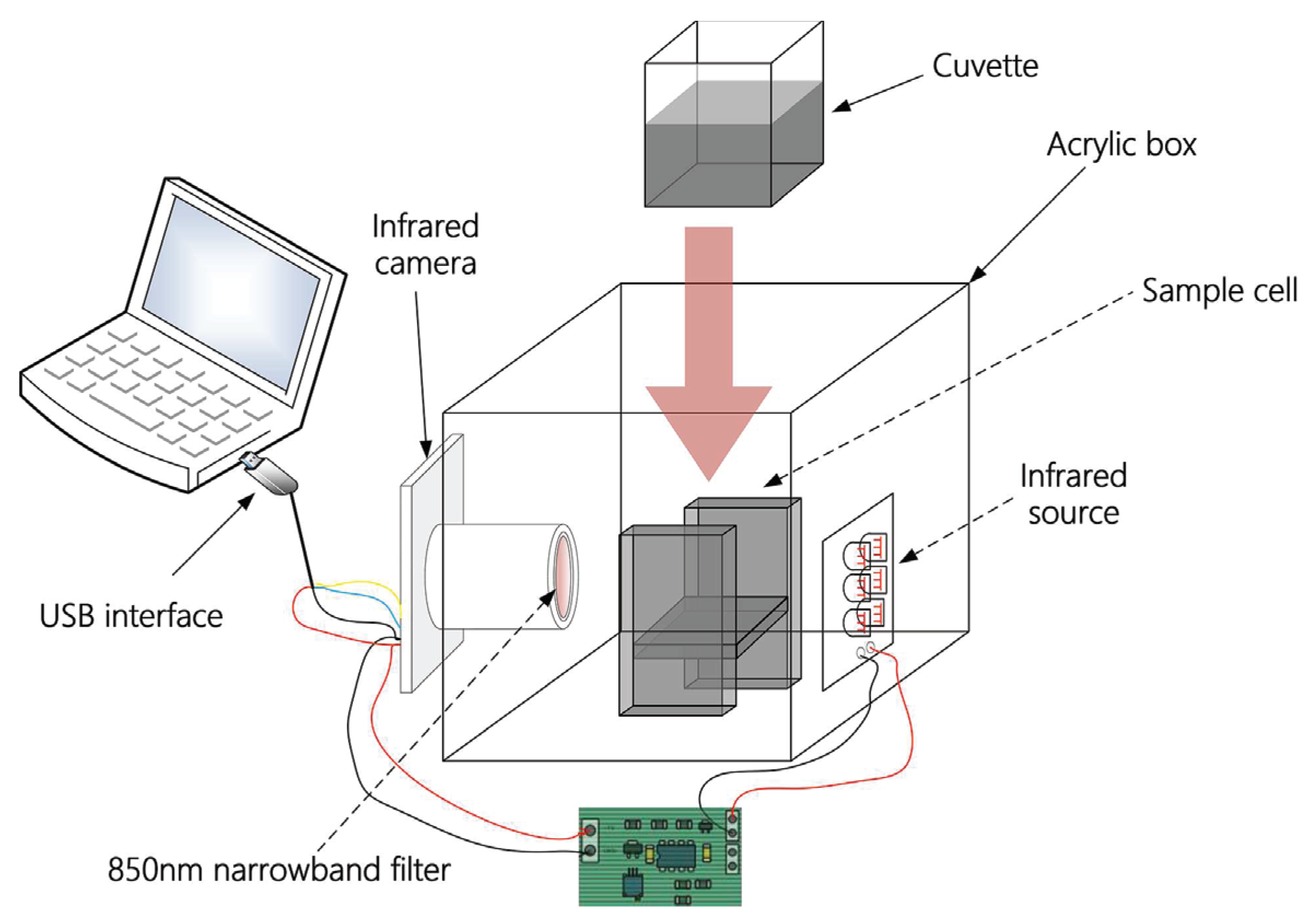
Applied Sciences Free Full Text Determination Of Vitamin C In Foods Using The Iodine Turbidimetric Method Bined With An Infrared Camera Html
| Title: Applied Sciences Free Full Text Determination Of Vitamin C In Foods Using The Iodine Turbidimetric Method Bined With An Infrared Camera Html Consider The Following Potential Sources Of Error In The Titration |
| Format: PDF |
| Number of Views: 3190+ times |
| Number of Pages: 166+ pages |
| Publication Date: June 2017 |
| Document Size: 1.8mb |
| Read Applied Sciences Free Full Text Determination Of Vitamin C In Foods Using The Iodine Turbidimetric Method Bined With An Infrared Camera Html |
 |
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: Google Sheet |
| Number of Views: 9210+ times |
| Number of Pages: 136+ pages |
| Publication Date: January 2020 |
| Document Size: 810kb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: Google Sheet |
| Number of Views: 7162+ times |
| Number of Pages: 45+ pages |
| Publication Date: June 2020 |
| Document Size: 5mb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: PDF |
| Number of Views: 7190+ times |
| Number of Pages: 85+ pages |
| Publication Date: July 2021 |
| Document Size: 3.4mb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |
Lab 9 Titrations
| Title: Lab 9 Titrations Consider The Following Potential Sources Of Error In The Titration |
| Format: Doc |
| Number of Views: 8180+ times |
| Number of Pages: 196+ pages |
| Publication Date: June 2019 |
| Document Size: 2.8mb |
| Read Lab 9 Titrations |
 |
Titration Lab Ap Chemistry Shelly Oh
| Title: Titration Lab Ap Chemistry Shelly Oh Consider The Following Potential Sources Of Error In The Titration |
| Format: PDF |
| Number of Views: 9195+ times |
| Number of Pages: 17+ pages |
| Publication Date: June 2020 |
| Document Size: 2.1mb |
| Read Titration Lab Ap Chemistry Shelly Oh |
 |
Over titrate the solution with a few ml of titrant and record the electrode potential. H if it would have caused your calculated value for Molarity of NaOH to come out too high L if it would have caused it to come out too low. Here are some common errors in titration.
Here is all you need to learn about consider the following potential sources of error in the titration Ralph Olliges Webster University. H if it would have caused your calculated value for Molarity of NaOH to come out too high L if it would have caused it to come out too low. Consider each of the following potential error sources. Lab 9 titrations titration lab ap chemistry shelly oh titration techniques in the food industry new food magazine mini titrator for measuring titratable alkalinity in water and wastewater hi84531u 01 applied sciences free full text determination of vitamin c in foods using the iodine turbidimetric method bined with an infrared camera html chemistry titrations university of birmingham Consider each of the following potential error sources.
0 Comments